Estrutura e fotoluminescência do germanato de magnésio contendo manganês tetravalente como cátion ativador
DOI:
https://doi.org/10.63595/vetor.v35i1.18365Palavras-chave:
Cerâmicas, Difração de raios-X, Fotoluminescência, Mn4+Resumo
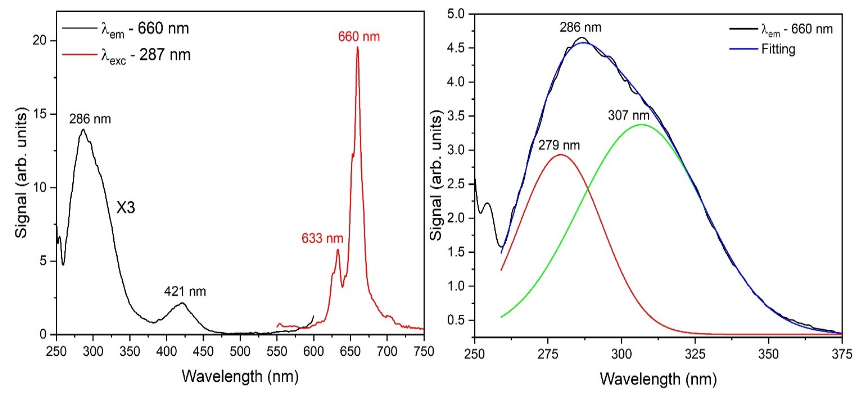
Apresentamos a síntese pelo método do acetato do composto germanato de magnésio (Mg2GeO4) contendo Mn4+ como cátion ativador de transições ópticas. A estrutura cristalina do composto foi caracterizada através de medidas de difração de raios-X em conjunto com o método de Rietveld, que confirmaram a formação do composto pretendido. Medidas de espectroscopia de fotoluminescência mostram uma emissão na região do vermelho. Sob excitação de 287 nm, o espectro de emissão exibe um pico intenso em 660 nm, acompanhado de diversas estruturas. As transições ópticas foram identificadas e confirmam a ocupação do Mn4+ em simetria octaédrica. A partir dos espectros ópticos os parâmetros de campo cristalino Dq, B e C foram calculados. O tempo de vida da emissão é da ordem de 2,8 ms, compatível com a transição eletrônica proibida por spin 2E(2G) → 4A2(4F) atribuída à emissão em 660 nm. Os resultados indicam que a amostra foi sintetizada com sucesso e as suas propriedades ópticas podem ser aproveitadas em dispositivos que operam com emissão na faixa do vermelho no espectro visível.
Downloads
Referências
K. Mahmood, J. Jacob, A. Rehman, A. Ali, U. Rehaman, N. Amin, S. Ikram, A. Ashfaq, and S. Hussain, “Modulation of thermoelectric properties of Mg2GeO4 thin films by controlling the growth process,” Ceramics International, vol. 45, no. 15, pp. 18701–18703, 2019. Available at: https://doi.org/10.1016/j.ceramint.2019.06.095
H. M. Yang, J. X. Shi, H. B. Liang, and M. L. Gong, “A novel red phosphor Mg2GeO4 doped with Eu3+ for PDP applications,” Materials Science and Engineering: B, vol. 127, no. 2–3, pp. 276–279, 2006. Available at: https://doi.org/10.1016/j.mseb.2005.10.014
C. X. Chen, S. P. Wu, and Y. X. Fan, “Synthesis and microwave dielectric properties of B2O3- doped Mg2GeO4 ceramics,” Journal of Alloys and Compounds, vol. 578, pp. 153–156, 2013. Available at: https://doi.org/10.1016/j.jallcom.2013.05.038
S. Deng, X. Qu., X. Wang, Y. Xiao, G. He, K. Liu, Q. Li, Z. Dai, X. Chen, and H. Zhou, “Solid-phase reaction mechanism and microwave dielectric properties of Mg2GeO4-MgAl2O4 composite ceramics,” Ceramics International, vol. 48, no. 21, pp. 31890–31895, 2022. Available at: https://doi.org/10.1016/j.ceramint.2022.07.122
J. Yang, Y. Zhou, H. Ming, E. Song, and Q. Zhang, “Site-selective occupancy of Mn2+ enabling adjustablered/ near-infrared multimode luminescence in olivine for dynamic anticounterfeiting and encryption,” Applied Electronic Materials, vol. 4, no. 2, pp. 831–841, 2022. Available at: https://doi.org/10.1021/acsaelm.1c01182
H. M. Yang, Z. Wang, M. L. Gong, and H. Liang, “Luminescence properties of a novel red emitting phosphor, Mg2GeO4:Sm3+,” Journal of Alloys and Compounds, vol. 488, no. 1, pp. 331–333, 2009. Available at: https://doi.org/10.1016/j.jallcom.2009.08.123
X. Wang, P. Li, M. G. Brik, X. Li, L. Li, and M. Peng, “Thermal quenching of Mn4+ luminescence in SrAl12O19:Mn4+,” Journal of Luminescence, vol. 206, pp. 84–90, 2019. Available at: https://doi.org/10.1016/j.jlumin.2018.10.044
A. M. Srivastava, M. G. Brik, C.G. Ma, W. W. Beers, W. E. Cohen, and M. Piasecki, “Effect of covalence and degree of cation order on the luminous efficacy of Mn4+ luminescence in the double perovskites, Ba2BTO6 (B = Y, Lu, Sc),” The Journal of Physical Chemistry Letters, vol. 15, no. 15, pp. 4175–4184, 2024. Available at: https://doi.org/10.1021/acs.jpclett.4c00205
W. Zou, R. Zhu, X. Zhang, Y. Li, J. Zuo, P. He, J. Zhang, W. Wang, J. Peng, and X. Ye, “Enhancing the luminescence of Mn4+-doped double perovskite oxide Ca2LuNbO6 phosphor by utilizing A-site cationic fluoride as a flux,” Ceramics International, vol. 50, no. 13, pp. 22627–22636, 2024. Available at: https://doi.org/10.1016/j.ceramint.2024.03.364
S. Adachi, “Photoluminescence properties of Mn4+-activated oxide phosphors for use in white-LED applications: A review,” Journal of Luminescence, vol. 202, pp. 263–281, 2018. Available at: https://doi.org/10.1016/j.jlumin.2018.05.053
H. Liu; J. Liu, B. Sun, Z. Zhang, C. Jiao, D. Sun, L. Zhang, and Y. Zhang, “Ca2LaTaO6:Bi3+/Mn4+ phosphors with high brightness far-red emitting and luminescence enhancement for plant growth LED lights and temperature sensor,” Inorganic Chemistry, vol. 63, pp. 5365–5377, 2024. Available at: https://doi.org/10.1021/acs.inorgchem.3c03939
M. Peng, X. Yin, P. Tanner, M. G. Brik, and P. Li, “Site occupancy preference, enhancement mechanism, and thermal resistance of Mn4+ red luminescence in Sr4Al14O25:Mn4+ for warm WLEDS,” Chemistry of Materials, vol. 27, no. 8, pp. 2938–2945, 2015. Available at: https://doi.org/10.1021/acs.chemmater.5b00226
F. Xue, Y. Hu, L. Chen, H. Wu, G. Ju, T. Wang, and L. Yang, “A novel rare-earth free red long-persistent phosphor: Mg2GeO4:Mn4+,” Ceramics International, vol. 43, no. 17, pp. 15141-15145, 2017. Available at: https://doi.org/10.1016/j.ceramint.2017.08.044
B. H. Toby, “R factors in Rietveld analysis: How good is good enough?,” Powder diffraction, vol. 21, no. 1, pp. 67–70, 2006. Available at: https://doi.org/10.1154/1.2179804
N. D. C. Santana, A. López, L. P. Sosman, and S. S. Pedro, “Photoluminescence of tetrahedrally coordinated Co2+ in magnesium titanate,” Optical Materials, vol. 134, pp. 113119, 2022. Available at: https://doi.org/10.1016/j.optmat.2022.113119
J. Rodríguez-Carvajal, “Recent advances in magnetic structure determination by neutron powder diffraction,” Physica B: Condensed Matter, vol. 192, no. 1–2, pp. 55–69, 1993. Available at: https://doi.org/10.1016/0921-4526(93)90108-I
K. Momma and F. Izumi, “VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data”, Journal of Applied Crystallography, vol. 44, no. 6, pp. 1272–1276, 2011. Available at: https://doi.org/10.1107/s0021889811038970
H. Cai, S. Liu, Z. Song, and Q. Liu, “Tuning luminescence from NIR-I to NIR-II in Cr3+ -doped olivine phosphors for nondestructive analysis,” Journal of Materials Chemistry C, vol. 9, no. 16, pp. 5469–5477, 2021. Available at: https://doi.org/10.1039/D1TC00521A
B. Jiang, B. Lou, Q. Liu, J. Zhang, F. Chi, and J. Zhang, “Investigation on the valence state stability and optical properties of Mg2GeO4:Cr,” Optical Materials, vol. 150, pp. 115136, 2024. Available at: https://doi.org/10.1016/j.optmat.2024.115136
E. H. H. Hasabeldaim, H. C. Swart, and R. E. Kroon, “Luminescence and stability of Tb doped CaF2 nanoparticles,” Royal Society of Chemistry, vol. 13, no. 8, pp. 5353–5366, 2023. Available at: https://doi.org/10.1039/d2ra07897j